I- Disorders of water-electrolytes metabolism
1. Water balance
A. Water content and distribution
Intracellular fluid (ICF)):
The fluid within cells.
Extracellular fluid (ECF):
The fluid outside the cells = interstitial fluid + intravascular fluid.
Body fluid: water + solutes
Accounts for 60% of body weight?
Body fluid varies with age, sex and the amount of body fat:
age ↑ → body fluid ↓
Adult females have a lower proportion of body fluid than the males of same age
fat ↑ body fluid ↓
B. Water daily balance
2. Electrolytes in Body fluid
--Na+ requirement is about 4-6g/day;
--mainly gain from daily salt supplement;
--About 90% is eliminated in the urine.
-- Characteristics of renal Na+ excretion:
High intake high excretion,
Low intake low excretion,
No intake no excretion.
-- Plasma [Na+]: 130~150mmol/L
3. Osmotic pressure in the body fluid
- Osmotic pressure of a solution depends on the amount of dissolved molecules or ions.
- Plasma osmotic pressure contains colloid osmotic pressure and crystal osmotic pressure.
Osmosis and Osmotic Pressure
When a semi-permeable membrane (a membrane that allows solvent molecules to flow through but not the solute particles) separates two solutions of different concentrations, there will be a net flow of solvent molecules from the solution where its
concentration is lower to the solution where its concentration is higher. This phenomenon is called Osmosis and driving pressure is called as Osmotic pressure.
Osmotic pressure:
Crystal osmotic pressure is formed by a lot of
small molecular weight materials, such as
electrolyte,Glucose, BUN and so on.
Colloid osmotic pressure is formed by large
molecular weight materials such as proteins.
>Osmotic pressure of ECF is roughly equivalent to ICF.
>Water moves from areas of low osmolatity to areas of high osmolatity.
>Normal plasma osmotic pressure is 280~ 310 mOsm/L.
Functions of electrolytes:
1.Maintaining the osmotic and acetic-alkali equilibrium.
2. Maintaining the resting membrane potential and generating the active membrane potential.
3. Taking part in metabolism and physiologic action.
Functions of body water:
- It is essential to metabolism of the body.
- It acts as a transport vehicle.
- It is a good lubricant.
- It is necessary for temperature regulation.
4. Regulation of water-salt metabolism
--Water balance is regulated primarily by antidiuretic hormone (ADH) and the perception of thirst;
-- Sodium balance is regulated by aldosterone.
Regulating mechanism
Thirst center
The regulation of ADH
The regulation of RAAS
II. Disorders of water-sodium metabolism
Dehydration
An excessive loss of body fluid resulting from various causes is termed dehydration.
1. Hypertonic dehydration
(1) Concept:
The dehydration in which water loss is in excess of Na+ loss and remaining ECF of the body is hypertonic is termed hypertonic dehydration.
Characteristics:
a. loss of water > loss of sodium;
b. plasma Na+ > 150mmol/L;
c. plasma osmotic pressure > 310mOsm/L.
(2) Causes:
A: Decreased water intake:
a. Inability to drink or loss of thirst:
- difficulty in swallowing: esophageal cancer
- unconsciousness: coma
- brain injury: impaired thirst sensation
b. Unavailableness of water:
e.g. desert district: desert travelling ocean accident; war, et al.
B: Increased water output:
Body fluid lose from: skin, lungs, GI, kidney
a. skin and lungs:
e.g. profuse sweating, fever
b. GI tract: gastrointestinal track (GI)
e.g. diarrhea, vomiting
c. Kidneys:
e.g. DM:
insipidus: central defect in ADH production or secration a renal insensitivity to ADH
(3) Effects on the body
Clinical characteristics:
Obvious thirst; mainly intracellular dehydration; a few patients occur circulatory failure (shock) at early stage.
A. Compensation process:
B. Decompensation (Clinical Manifestations)
Dehydration fever:
Serious dehydration may lead to increased body temperature due to decreased evaporation from skin and impaired temperature regulatory function.
Degree of hypertonic dehydration
Principles of treatment
A. To treat primary disease;
B. To supply water:
--drink water,
--transfuse 5% glucose first, then 0.9% NaCl.
2. Hypotonic dehydration
(1) Concept:
The dehydration in which sodium loss is in excess of water loss and remaining ECF of the body is hypotonic is termed hypotonic dehydration.
Characteristics:
a. loss of sodium > loss of water;
b. plasma Na+ < 130mmol/L;
c. plasma osmotic pressure < 280mOsm/L.
(2) Causes:
The most common cause:
fluid loss + replacement of water or intravenous 5% glucose only.
Body fluid lose from:
A: GI tract: e.g. vomiting, diarrhea.
B: skin: perfuse sweating, a large area of burn.
C. Kidneys:
a. Administer some diuretics for a long time:
b. Lack of aldosterone: e.g. Addison’s disease
c. Intrinsic renal disease:
e.g. acute renal failure
The conditions of a and b can cause excessive renal lose of sodium.
(3) Effects on the body
Clinical characteristics:
Mainly extracellular dehydration; circulatory failure (shock) easily occurs at early stage.
A. Compensation process:
B. Decompensation (Clinical Manifestations)
Degree of hypotonic dehydration
Principles of treatment
A. To treat the causes of the disease;
B. Administer sodium solution, either orally
or intravenously.
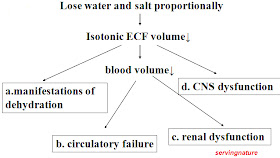
3. Isotonic dehydration
(1) Concept:
The dehydration in which water loss is equal to sodium loss and remaining ECF of the body is isotonic is termed isotonic dehydration.
Characteristics:
a. water and sodium lost proportionally;
b. plasma Na+ = 130-150mmol/L;
c. plasma osmotic pressure = 280-310mOsm/L.
(2) Causes:
A. Acute serious vomiting or diarrhea:
B. Skin burn in a large area:
C. Draw a large amount of ascites:
D. Paralytic ileus:
(3) Effects on the body
A. Compensation process:
B. Decompensation (Manifestations)
Principles of treatment
A. To treat original disease;
B. Supply isotonic fluid.
Distribution of body fluid in dehydration